|
Luisa Maia received her Ph.D. degree in Pharmaceutical and Clinical Biochemistry from the Universidade de Lisboa (Portugal), for work on the characterisation of molybdenum-containing enzymes relevant to Human Health & Disease.
L. Maia "core" research interests are focused on the kinetic, spectroscopic and mechanistic characterisation of metalloenzymatic systems and on their physiological functions and biotechnological applications. Her research efforts have been mainly dedicated to the study of two enzymatic systems:
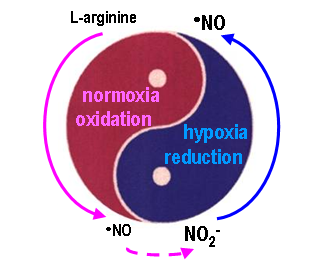
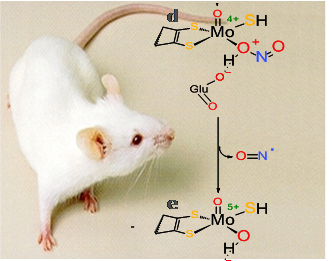
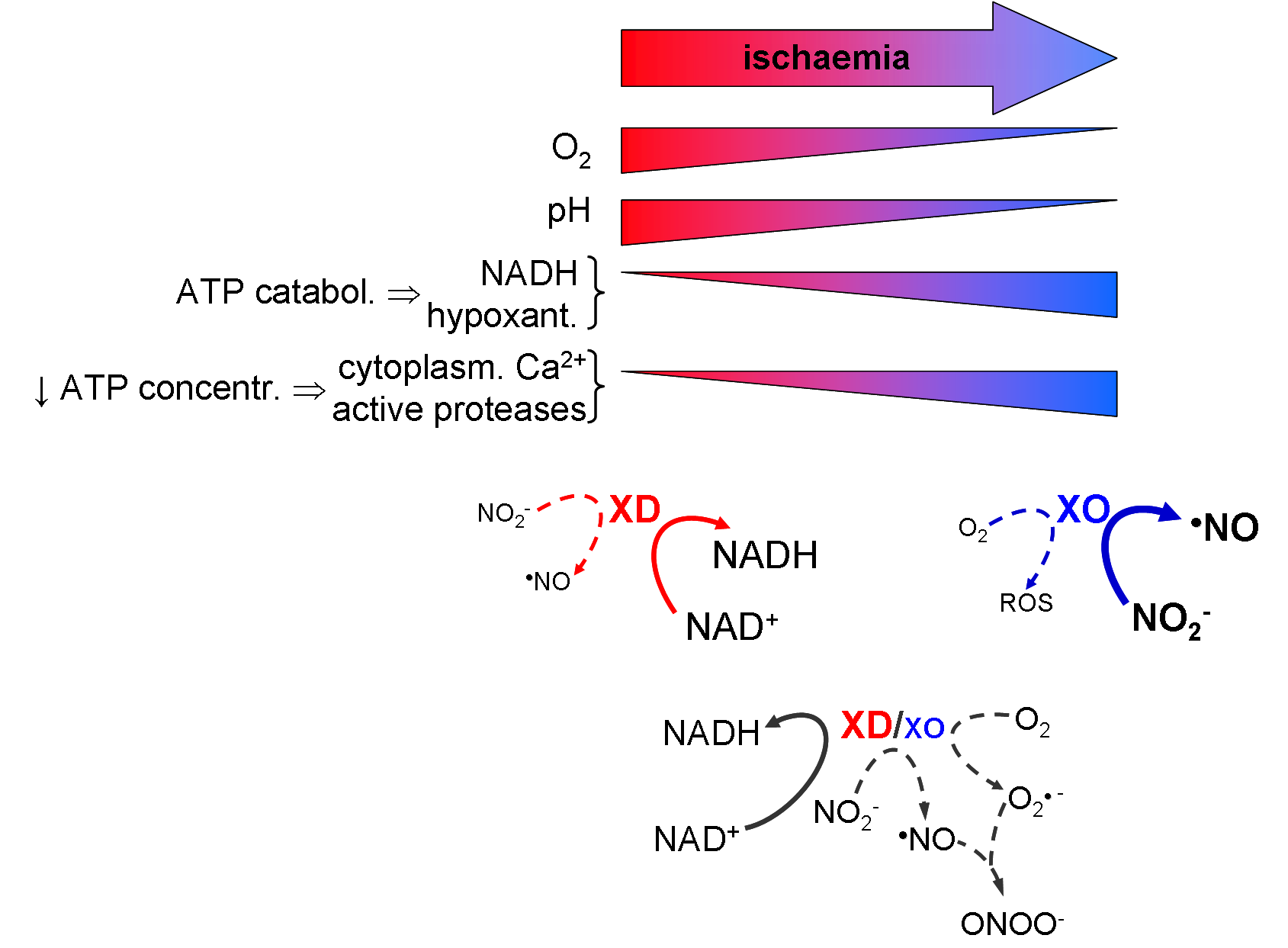
(i) the new signalling nitrate-nitrite-nitric oxide pathway and its implications to human health and disease and
(ii) formate dehydrogenase enzymes and their exploitation for carbon dioxide utilisation (CDU) and bioproduction of energy.
Both systems are aligned with the UN 2030 Agenda for Sustainable Development (Goals 3, 7 and 13).
L. Maia has been also characterising other interesting biological and inorganic systems containing metals or involved in the formation/consumption of radical species.
.
In more detail, L. Maia research interests are:
Human signalling pathways | Gasotransmitters | ROS/RNS
• Nitric oxide (•NO) metabolism - human and bacterial
• Nitric oxide sensing
• Mechanisms of disease
• Reactive oxygen and nitrogen species (ROS/RNS) metabolism
CDU - Enzymatic systems for carbon dioxide utilisation
• Reduction to formate
• Kinetic and mechanistic studies
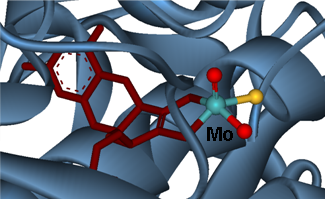
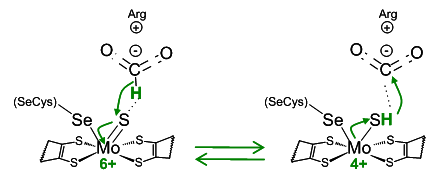
Structure-activity relationships in metalloproteins
(molybdenum, copper, haem and non-haem-iron)
• Spectroscopic and kinetic characterisation of metalloproteins and small proteins mimetics
• Spectroscopic and kinetic characterisation of model complexes
• Theoretical mechanistic studies of enzymatic mechanisms
• Spectroscopic and kinetic characterisation of compounds with antioxidant activity
New catalytic activities of molybdoenzymes and other metalloproteins
• Kinetic and mechanistic studies and in situ studies,
in mammalian, plant and bacterial enzymes
Spectroscopic characterisation of biological and inorganic metallic systems
• Kinetic and mechanistic studies and in situ studies
EPR characterisation of biological and inorganic systems
involved in the formation/consumption of radical species
• Reactive oxygen and nitrogen species (ROS/RNS)
• Other radical species
FIELDS OF KNOWLEDGE: Biochemistry; Enzymology; Bioinorganic Chemistry; Spectroscopy
EXPERTISE: protein production, biochemical & functional characterisation - kinetics, mechanisms, spectroscopy
Curriculum vitae Synopsis (as of December/2023):
L. Maia has a total of 55 publications: 39 articles in international journals with scientific refereeing (all in Q1 journals; 1 published as a 'Hot Paper'; accumulated 5-year Impact Factor > 250), 7 book chapters and 9 conferences abstracts. She is corresponding author of 17 publications and first author of 25, having a h-index of 18 (>1,260 citations; SCOPUS ID 23005391000) and i10-index of 28 (>1,690 citations; GoogleScholar ID ZtMrILoAAAAJ). L.Maia is also co-editor of 2 Books (Royal Society of Chemistry and Springer) and guest-editor of 2 special issues (J. Biol. Inorg. Chem. (gathered 22 articles) and Front. Chem. Biol. (ongoing)). In addition, L.Maia authored 5 articles and 1 book of science dissemination in portuguese language. L. Maia actively participates in international conferences, having presented 26 oral communications, of which 7 were Invited Comm.. In national conferences, she has presented 14 oral communications, of which 4 were Invited and 1 Keynote. Presented >60 posters. L. Maia also acted as Organising Committee Member in 7 conferences and as Session Chair in 6. She is President of the Portuguese SPQ Inorganic and Bioinorganic Chemistry Division and Chair of the upcoming "2024 Inorganic & Bioinorganic Chemistry Conference, IBCC2024". She has been actively participating in several (8) R&D Projects, contributing to secure ≈ 1.9M Euros and has experience in students and Post-Doc supervision (2 Post-Doc, 1 PhD student, 5 Masters, 7 Master students and 17 Undergraduate students). She has been also teaching (average of 25h/semester) at Bachelor and Master Degree levels and, since 2010, mentoring several students and PhD-holders in EPR spectroscopy. L. Maia has also been enthusiastically organising and participating in different outreach activities.
LINKS:
 LinkTree: LuisaBMaia (here) LinkTree: LuisaBMaia (here)
 Scopus ID: 23005391000 (here) Scopus ID: 23005391000 (here)
 Ciência ID: EF19-47FC-7FA9 (here) Ciência ID: EF19-47FC-7FA9 (here)
 ORCID ID: 0000-0002-6901-6591 (here) ORCID ID: 0000-0002-6901-6591 (here)
 Researcher ID: F-8106-2011 (here) Researcher ID: F-8106-2011 (here)
 Google Scholar (here) Google Scholar (here)
 NOVA Research Portal (here) NOVA Research Portal (here)
 ResearchGate (here) ResearchGate (here)
 @LuisaBMaia @LuisaBMaia
|
|